“Juice” is a slang term sometimes used for electricity. Batteries are made up of one or more cells. Cells often consist of two different materials in a solution and connected to each other by a wire. In this experiment, you will study some basic principles of cells using the juice of a lemon or other fruit as the cell solution. You will place pieces of two different materials into the fruit, and a computer will be used to measure and display the voltages produced.
In this experiment, students will
- Learn the basic components of an electric cell.
- Develop methods to study the different variables involved.
- Discover which combinations produce the best voltage.
computer (if needed)
copper wire (Cu)
Vernier interface
galvanized nail (Zn)
Vernier Voltage Probe
various metal samples
2 alligator clips
various fruits
a lemon or other fruit
paper towels
scalpel
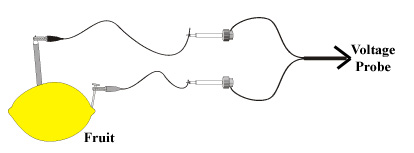
Procedure:
- The instructor will demonstrate a basic voltaic cell as shown in Figure 1. He/she will insert two different metals into a lemon or other fruit then connect them to a voltage probe connected to an interface.
- The instructor will launch Logger Pro or Logger Lite software on a computer, or LabQuest app on a LabQuest. When using LabQuest, teachers are recommended to use LabQuest Emulator software and a projector so the whole class can observe the resulting voltage.
- Students will observe and make note of the voltage that is produced.
- Students will then identify the key components of the arrangement and then how they could go about changing each. The instructor will ask for class members to contribute their suggestions and record them in a highly visible manner. (Alternatively, small groups could develop their own set of changes to study, then participate in the whole-class discussion.)
- Groups will either select or be assigned to study one of the identified variables in depth. They will keep careful track of the changes they make and the results of each.
- Upon completion of the experimentation, the groups will each make concise presentations of their work that will be shared by the entire class.
- Assessment can be completed either through written reports or questions on a quiz or other examination.
- Measure the voltage of cells connected in series and in parallel.
1. Some variables that might be investigated:
- Direction of the leads on the Voltage Probe
- Types of metals
- Distance of separation of the electrodes
- Type of fruit
- Compare juices to fruit
- Physical size of the electrodes
2. Online research could be done to learn the materials used in commercial electric cells. Other research could be done to learn better how Volta created his initial voltaic piles.
3. A
possible data table might resemble the following:
| Trial # |
Red Material* |
Black Material* |
Fruit or Other |
Voltage |
| 1 |
||||
| 2 |
||||
| 3 |
* - What material is connected to the red electrode and what material is connected to the black electrode of the Voltage Probe
4. Some additional materials that should available include steel wool to clean electrodes and a weak acid (vinegar) to also clean electrodes. Water is also needed to rinse the vinegar off if it is used.
5. Suggestions for metals include copper, zinc, iron, aluminum, nickel, lead, magnesium and various alloys. Carbon is another material that proves useful and can be obtained as pencil “lead”. Galvanized nails have zinc coating while non-galvanized nails are primarily iron.
Click here for MS Word document: lemon.doc
Clarence Bakken
October 2011