|
In Physical Science and Chemistry, students often
study the characteristics behind the Periodic Table.
LabQuest has a routine built into it that will aid in
discovering and characterizing what we call "periodicity".
New File
Start LabQuest app by turning LabQuest 2 on. If your LabQuest 2 is already on, we recommend you create a new file by tapping on File > New. Either save or discard any data that is already in the LabQuest memory.
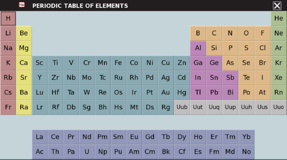
Periodic Table
- Tap on the "Home" icon in the lower task bar,
 . Select Periodic Table from
the menu that appears.
. Select Periodic Table from
the menu that appears. - Tap on any of the elements in the table. Note the information that is available for each of the elements.
- You can put the information away fror the current element by tapping the "X" in the upper right-hand corner, then select another element if you wish.
- To put away the whole Periodic Table, tap the "X" in the upper right-hand corner above the table.
Plotting Table Data
- Tap File > Open >
periodic_table_data.qmbl then "Open"
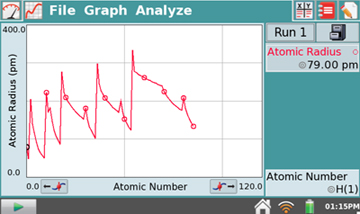
- Tap on the Graph icon
 and you
will be presented with a graph of Atomic Radius (pm) versus
Atomic Number. "pm" stands for picometer, 10-12
m.
and you
will be presented with a graph of Atomic Radius (pm) versus
Atomic Number. "pm" stands for picometer, 10-12
m. - Tapping on the graph results in the display of the specific atomic radius and corresponding atomic number and symbol for the position you tapped.
- If you want to advance ahead by a single atomic number,
tap the right-hand arrow icon,
 . If you
want to go backward a single atomic number, tap the
left-hand arrow button.
. If you
want to go backward a single atomic number, tap the
left-hand arrow button. - Which elements form the peaks in the Atomic Radius vs.
Atomic Number graph? Where are these elements in the
Periodic Table? What does this graph indicate about the
sizes of atoms as you move through the Periodic Table?
Other Plots
LabQuest 2 can display other quantities for study. Tap on the y-axis label (Atomic Radius (pm)) and choose from the various quantities that are shown you -- Melting Point, Boiling Point, Density, etc. In this way you can study other important qualities of elements as you move through the Periodic Table.
Even More
- Change the y-axis to First Ionization Energy, the energy needed to add or remove an electron from the atom. Tap on the x-axis label and change it to Atomic Radius, the size of the atom.
- The graph will be very busy with lines, but we can remove them by tapping on Graph > Graph Options. On the lower left-hand side uncheck the box for "Connect Points" and also the box for "Point Symbols".
- When you tap on "OK" you will see a scatter plot of the data for Ionization Energy vs. Atomic Radius. What does this graph tell you?
The use of this powerful tool, which is built into LabQuest, is now in the hands of the instructors.
Also see: http://www.vernier.com/innovate/periodic-table-graphing/
Click here for MS Word file of this activity
Click here for pdf file of this activity
Click here for specific instructions for LabQuest 1.
C. Bakken
March 2012